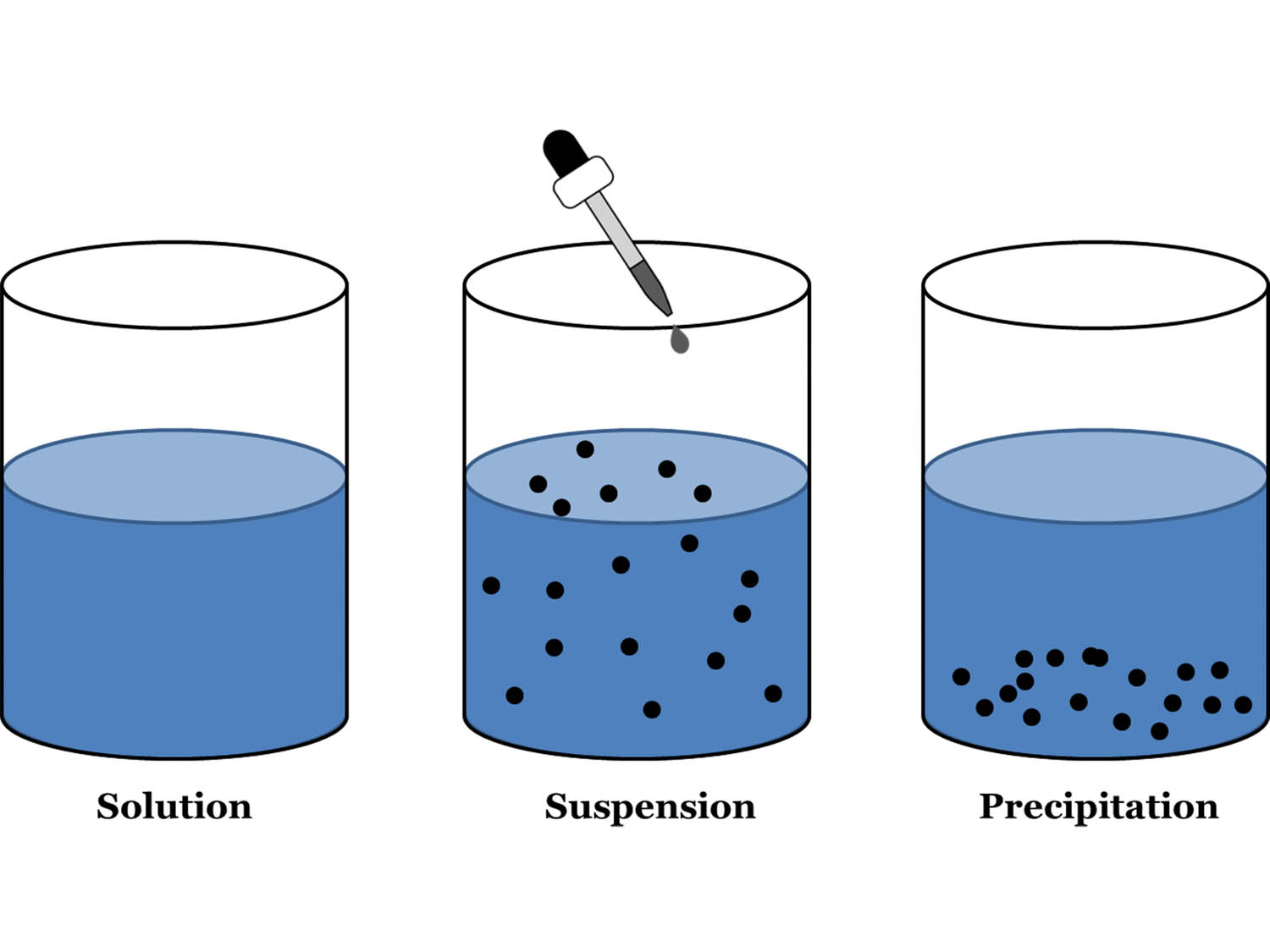
Homogeneous Suspension Mixture . No matter where you sample the. Explain the significance of the statement like. The dissolving agent is the solvent. — a suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. A suspension is a heterogeneous mixture that does not dissolve and the different parts. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of. — a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. — a solution is a homogeneous mixture where one substance dissolves into another and stays mixed together. — learning objectives. The substance that is dissolved is the solute. — a solution is a homogeneous mixture of two or more components.
from byjus.com
The dissolving agent is the solvent. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of. — a solution is a homogeneous mixture of two or more components. No matter where you sample the. — a solution is a homogeneous mixture where one substance dissolves into another and stays mixed together. — learning objectives. Explain the significance of the statement like. The substance that is dissolved is the solute. — a suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes.
Suspensions (Chemistry) Definition, Properties, Examples with Videos
Homogeneous Suspension Mixture — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. — a solution is a homogeneous mixture where one substance dissolves into another and stays mixed together. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. Explain the significance of the statement like. The substance that is dissolved is the solute. No matter where you sample the. — a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. — learning objectives. — a suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of. The dissolving agent is the solvent. — a solution is a homogeneous mixture of two or more components. A suspension is a heterogeneous mixture that does not dissolve and the different parts.
From stock.adobe.com
Solutions, suspension, precipitate. Solubility homogeneous mixture Homogeneous Suspension Mixture — a suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. — learning objectives. The substance that is dissolved is the solute. The dissolving agent is the solvent. — a suspension is a heterogeneous mixture in which some of the particles. Homogeneous Suspension Mixture.
From www.slideshare.net
Properties of suspensions Homogeneous Suspension Mixture — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. — a solution is a homogeneous mixture where one substance dissolves into another and stays mixed together. — a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. — a suspension is. Homogeneous Suspension Mixture.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Homogeneous Suspension Mixture — a solution is a homogeneous mixture where one substance dissolves into another and stays mixed together. The substance that is dissolved is the solute. — a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. — a suspension is defined as a homogenous mixture of particles with a. Homogeneous Suspension Mixture.
From www.slideserve.com
PPT Topics States of Matter Pure Substances Mixtures Physical and Homogeneous Suspension Mixture No matter where you sample the. — a solution is a homogeneous mixture where one substance dissolves into another and stays mixed together. The substance that is dissolved is the solute. A suspension is a heterogeneous mixture that does not dissolve and the different parts. — a suspension is defined as a homogenous mixture of particles with a. Homogeneous Suspension Mixture.
From stock.adobe.com
Solutions, suspension, precipitate. Solubility homogeneous mixture Homogeneous Suspension Mixture — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. — a suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. — a suspension is a heterogeneous mixture in which some of the particles settle. Homogeneous Suspension Mixture.
From smartclass4kids.com
What is Mixture, Homogeneous Mixture, Heterogeneous Mixture with Examples Homogeneous Suspension Mixture No matter where you sample the. — learning objectives. — a suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. A suspension is a heterogeneous. Homogeneous Suspension Mixture.
From in.pinterest.com
Solutions, suspensions, and colloids Suspension mixture, Body diagram Homogeneous Suspension Mixture A suspension is a heterogeneous mixture that does not dissolve and the different parts. The dissolving agent is the solvent. — a solution is a homogeneous mixture of two or more components. — a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. — learning objectives. — a. Homogeneous Suspension Mixture.
From dxobsnaxz.blob.core.windows.net
Soft Drink Is It A Homogeneous Mixture at Jimmie Guzman blog Homogeneous Suspension Mixture The dissolving agent is the solvent. — a suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. A suspension is a heterogeneous mixture that does not dissolve and the different parts. — learning objectives. No matter where you sample the. —. Homogeneous Suspension Mixture.
From diagramlibengelsitir.z13.web.core.windows.net
Homogeneous Mixture In Science Homogeneous Suspension Mixture — a solution is a homogeneous mixture where one substance dissolves into another and stays mixed together. — a solution is a homogeneous mixture of two or more components. — a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. — learning objectives. The substance that is dissolved. Homogeneous Suspension Mixture.
From www.gauthmath.com
Solved Classify the following as a heterogeneous mixture (suspension Homogeneous Suspension Mixture — a solution is a homogeneous mixture where one substance dissolves into another and stays mixed together. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. — a solution is a homogeneous mixture of two or more components. A suspension is a heterogeneous mixture that does not dissolve and the. Homogeneous Suspension Mixture.
From examples.yourdictionary.com
Examples of Homogeneous Mixtures Solid, Liquid and Gas YourDictionary Homogeneous Suspension Mixture suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of. The dissolving agent is the solvent. — a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. — a solution is a homogeneous mixture of two or more components. . Homogeneous Suspension Mixture.
From www.youtube.com
Skills 9b and 11 Mixtures, Suspensions, and Solutions YouTube Homogeneous Suspension Mixture — a solution is a homogeneous mixture of two or more components. — learning objectives. A suspension is a heterogeneous mixture that does not dissolve and the different parts. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of. The dissolving agent is the solvent. The substance that. Homogeneous Suspension Mixture.
From fixpartumbremeteorists.z13.web.core.windows.net
Homogeneous Mixture With Dissolved Particles Homogeneous Suspension Mixture — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. — a suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. — a solution is a homogeneous mixture of two or more components. A suspension. Homogeneous Suspension Mixture.
From www.scribd.com
Heterogeneous vs. Homogeneous Mixtures PDF Mixture Suspension Homogeneous Suspension Mixture — a solution is a homogeneous mixture where one substance dissolves into another and stays mixed together. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. — learning objectives. The dissolving agent is the solvent. suspensions and colloids are two common types of mixtures whose properties are in many. Homogeneous Suspension Mixture.
From www.slideserve.com
PPT Chapter 1314 Mixtures and Aqueous Solutions PowerPoint Homogeneous Suspension Mixture — a solution is a homogeneous mixture of two or more components. — a suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. — a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture. Homogeneous Suspension Mixture.
From dxojgzbqt.blob.core.windows.net
Suspension In Chemistry at Kirkham blog Homogeneous Suspension Mixture The dissolving agent is the solvent. — learning objectives. — a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. — a solution is a homogeneous mixture where one substance dissolves into another and stays mixed together. The substance that is dissolved is the solute. A suspension is a. Homogeneous Suspension Mixture.
From www.teachoo.com
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo Homogeneous Suspension Mixture No matter where you sample the. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. The dissolving agent is the solvent. A suspension is a heterogeneous mixture that does not dissolve and the different parts. — a suspension is a heterogeneous mixture in which some of the particles settle out of. Homogeneous Suspension Mixture.
From www.teachoo.com
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo Homogeneous Suspension Mixture — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. — learning objectives. — a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. Explain the significance of the statement like. A suspension is a heterogeneous mixture that does not dissolve and the. Homogeneous Suspension Mixture.